Molecular Machines
| 06. Oktober 2016The Nobel Prize in Chemistry 2016 was awarded to Jean-Pierre Sauvage, Sir Fraser J. Stoddart and Bernard L. Feringa for the design and synthesis of molecular machines. Freddy Kleitz, chemist at the University of Vienna, illustrates the relevance of this research area.
The achievements of the three scientists stem from the area of chemistry known as supramolecular chemistry (a term proposed by Jean-Marie Lehn, Nobel Prize laureate in 1987) and they branch into nanoscience and nanotechnology. Supramolecular chemistry has initially been defined as "the chemistry of the noncovalent bond". On the other hand, nanoscience is the science that studies the properties of matter that has at least one dimension of the order of a billionth of a metre (1 x 10-9), i.e. - a nano-metre. Accordingly, nanotechnology refers to the development of applications based on nanoscience.

About the author:
Freddy Kleitz is Professor of Inorganic Chemistry and Head of the Department of Inorganic Chemistry – Functional Materials at the Faculty of Chemistry of the University of Vienna. His research activities focus on designing functional nanoporous inorganic and organic-inorganic hybrid materials and exploring their properties as selective sorbents (e.g. for water treatment, extraction chromatography), as heterogeneous catalysts, and as biomedical materials. In particular, he is interested in the design and development of multifunctional and smart hybrid nanovehicles for targeted therapeutic applications. Jean-Pierre Sauvage was one of his teachers 20 years ago in Strasbourg. In their work, they share the common feature of trying to escape from equilibrium with "dynamic" hybrid material systems.
Miniaturised machines: Dream or reality
In our highly industrialised societies, we build life machines to perform a desired task every day, and these are necessary to support all our activities. Machines demonstrate well-regulated movements and perform specific functions with energy input. However, is it possible to create minuscule machines that would behave like macroscopic machines, but would have ultra-small dimensions? Is it possible to build a machine on the nanometre scale, that is, 1000 times thinner than a strand of hair? Or at the molecule level?
Such nanoscale "machines" or "nanomachines" already exist in nature and the human body is actually full of functional systems that could be regarded as single-molecule machines. Yet, designing and synthesising artificial super tiny machines, while being the dream of scientists for a long time still remain topics of fertile imagination.
Owing to the contributions of the three laureates, artificial molecular machines are now no longer science-fiction. The first nanomachines have been successfully developed and include for instance, tiny molecular elevators going up and down with pH changes, molecular-scale micro-electronic devices, and ultra-small motors. Obviously, we are still at the initial stage, however perspectives for future nanomachines designed at the molecular level and that are able to carry out desired operations, i.e. nanovehicles and nanorobots, are not so distant and may provide spectacular progress in many areas of research and technology. Potential uses are vast, such as new materials and systems for energy storage, electronics, sensors, nanomedicine, just to name a few.
From mechanically interlocked molecules to the first molecular motors
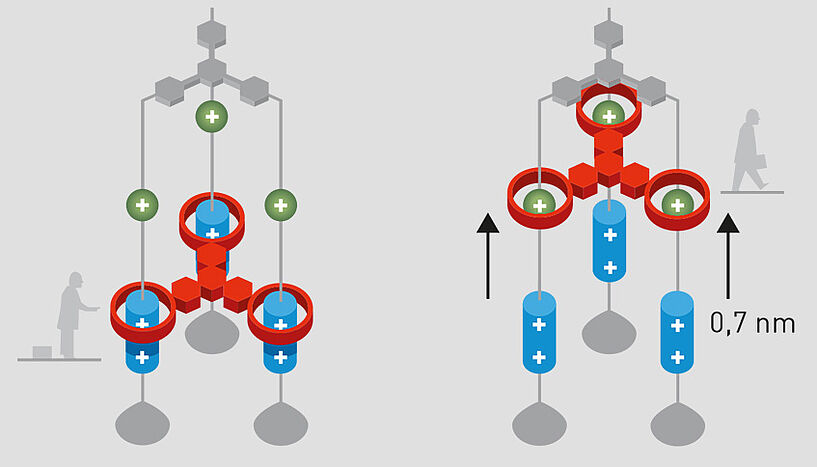
Fabricating these machines on the molecular scale (i.e. molecular machines) was not an easy task. First it required the development of innovative chemistry. Instead of typical molecules held together by strong bonds (named covalent bonds), the laureates efficiently designed and created mechanical bonds where molecules are interlocked, just like chains, without the atoms interacting strongly with each other.
With the Nobel Prize, Jean-Pierre Sauvage and Sir Fraser J. Stoddart are recognised for their pioneering work on the development of such supramolecular interlocked molecular systems, where the different components are linked through "mechanical" bonds. The particular systems developed by Jean-Pierre Sauvage in Strasbourg are viewed as the first step towards building a molecular machine. Remarkably, in 1994, Sauvage and his team built the first non-biological molecular machine. At about the same time, Stoddart created a ring-shaped molecule that was mechanically attached to a rod-shaped one. Here, the ring molecule was free to move along the rod-shaped molecule that thus acts as an axle. For the first time, Stoddart was also able to control the movement of the ring-like molecule along the axle. This is truly impressive as, typically, molecule motions are random.
These early developments were followed by many different molecular machines, including mobile elastic structures evocative of artificial muscles. Nevertheless, the third Nobel Prize laureate, Bernard Feringa, went a step further, still following a similar mechanical-bonding concept. In 1999, Bernard Feringa achieved a major breakthrough by producing the first molecular motor in which the molecules spin in one single direction. This movement mechanism was based on external stimuli-induced (e.g., heat, light) isomerisation of a double bond. From this progress, Feringa was then able to assemble a spectacular motorised single-molecule four-wheel-drive nanocar, with four molecular motors that function as the wheels. The wheels can spin and the nanocar moves forward along a metal surface. The scientists' dream came true.

Auch für den Chemiker Nuno Maulide vom Institut für Organische Chemie ist die Vergabe des Chemie-Nobelpreises für molekulare Maschinen "außergewöhnlich. Vor allem, weil es sich um ein erst im Entstehen befindliches Gebiet handelt". Normalerweise würde das Nobelpreiskomitee Arbeiten bevorzugen, die schon Anwendungen gefunden haben.
Künftig könnten die Mini-Maschinen auch in der Medizin eingesetzt werden. "Der Forschungsbereich ist unbedingt vielversprechend", so der Professor für Organische Synthese. Er sei zudem sehr erfreut darüber, dass der Preis im Bereich der Organischen Chemie vergeben wurde. Zum APA-Interview
Some prospects for energy and biological applications?
The three laureates designed and developed the molecular machines, mostly based on supramolecular chemistry of organic molecules. This offers scientists and researchers infinite new opportunities to generate ever more complex miniaturised machines and devices now. To illustrate future developments, one can cite the combination of molecular motors and organic polymers able to reversely store energy, which in my opinion, opens exciting perspectives for new batteries or energy conversion systems.
One other famous system has even been recently described as a so-called molecular robot, whose function is to link amino acid moieties. Furthermore, we can imagine multifunctional nanovehicles or such nanorobots, which could perhaps even travel the human body to achieve targeted therapeutic and diagnostic purposes. And we are not so far from there. Indeed, the research area of targeted drug delivery and therapy based on smart nanotransporters or nanomachines currently undergoes a remarkable expansion, owing to progress with molecular machinery. Nevertheless, most systems remain proof-of-concepts and the feasibility for real-world applications still needs to be demonstrated, so that molecular machines could become an integral part of our lives.
However, and most importantly, over the years the three chemist laureates have been able to effectively drive typical molecular systems "away from equilibrium" conditions, and this is precisely what life is actually doing. Out of equilibrium, which enables to collect and use energy, the molecules of life carry out specific and programmed tasks. As aptly recognised by the Nobel committee, molecular machines are designed following the same principles. This could therefore bring us greater understanding of biological systems and, ultimately, the ability to reproduce or repair the biological molecular machines. Certainly, we have merely witnessed the early phase of what could be achieved through miniaturisation of the machines at the molecular and nanometre levels. Thus, we should be looking forward to the incredible new developments ahead.
Freddy Kleitz is Professor of Inorganic Chemistry and Head of the Department of Inorganic Chemistry – Functional Materials at the Faculty of Chemistry of the University of Vienna.