Capturing brain activity with sculpted light
09. September 2013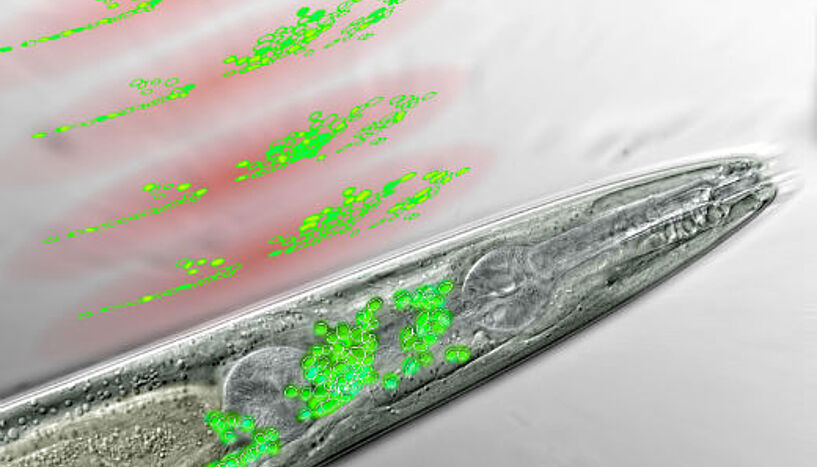
Frontal part of a nematode seen through a microscope. The neurons of the worm’s “brain” are coloured in green. Above are the discs of light generated by the WF-TeFo microscope, scanning the brain area and recording the activity of certain neurons (artist’s interpretation) (Copyright: IMP)
Researchers in Vienna develop new imaging technique to study the function of entire nervous systems Scientists at the Campus Vienna Biocenter (Austria) have found a way to overcome some of the limitations of light microscopy. Applying the new technique, they can record the activity of a worm’s brain with high temporal and spatial resolution, ultimately linking brain anatomy to brain function. The journal Nature Methods publishes the details in its current issue.
A major aim of today’s neuroscience is to understand how an organism’s nervous system processes sensory input and generates behavior. To achieve this goal, scientists must obtain detailed maps of how the nerve cells are wired up in the brain, as well as information on how these networks interact in real time.
The organism many neuroscientists turn to in order to study brain function is a tiny, transparent worm found in rotting soil. The simple nematode C. elegans is equipped with just 302 neurons that are connected by roughly 8000 synapses. It is the only animal for which a complete nervous system has been anatomically mapped.
Researchers have so far focused on studying the activity of single neurons and small networks in the worm, but have not been able to establish a functional map of the entire nervous system. This is mainly due to limitations in the imaging-techniques they employ: the activity of single cells can be resolved with high precision, but simultaneously looking at the function of all neurons that comprise entire brains has been a major challenge. Thus, there was always a trade-off between spatial or temporal accuracy and the size of brain regions that could be studied.
Scientists at Vienna’s Research Institute of Molecular Pathology (IMP), the Max Perutz Laboratories (MFPL), and the Research Platform Quantum Phenomena & Nanoscale Biological Systems (QuNaBioS) of the University of Vienna have now closed this gap and developed a high speed imaging technique with single neuron resolution that bypasses these limitations. In a paper published online in Nature Methods, the teams of Alipasha Vaziri and Manuel Zimmer describe the technique which is based on their ability to “sculpt” the three-dimensional distribution of light in the sample. With this new kind of microscopy, they are able to record the activity of 70% of the nerve cells in a worm’s head with high spatial and temporal resolution.
“Previously, we would have to scan the focused light by the microscope in all three dimensions”, says quantum physicist Robert Prevedel. “That takes far too long to record the activity of all neurons at the same time. The trick we invented tinkers with the light waves in a way that allows us to generate “discs” of light in the sample. Therefore, we only have to scan in one dimension to get the information we need. We end up with three-dimensional videos that show the simultaneous activities of a large number of neurons and how they change over time.” Robert Prevedel is a Senior Postdoc in the lab of Alipasha Vaziri, who is an IMP-MFPL Group Leader and is heading the Research Platform Quantum Phenomena & Nanoscale Biological Systems (QuNaBioS) of the University of Vienna, where the new technique was developed.
However, the new microscopic method is only half the story. Visualising the neurons requires tagging them with a fluorescent protein that lights up when it binds to calcium, signaling the nerve cells’ activity. “The neurons in a worm’s head are so densely packed that we could not distinguish them on our first images”, explains neurobiologist Tina Schrödel, co-first author of the study. “Our solution was to insert the calcium sensor into the nuclei rather than the entire cells, thereby sharpening the image so we could identify single neurons.” Tina Schrödel is a Doctoral Student in the lab of the IMP Group Leader Manuel Zimmer.
The new technique that came about by a close collaboration of physicists and neurobiologists has great potentials beyond studies in worms, according to the researchers. It will open up the way for experiments that were not possible before. One of the questions that will be addressed is how the brain processes sensory information to “plan” specific movements and then executes them. This ambitious project will require further refinement of both the microscopy methods and computational methods in order to study freely moving animals. The team in Vienna is set to achieve this goal in the coming two years.
Publication in Nature Methods:
Tina Schrödel, Robert Prevedel, Karin Aumayr, Manuel Zimmer und Alipasha Vaziri: Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. In: Nature Methods (September 2013).
DOI:10.1038/nmeth.2637
Max F. Perutz Laboratories
The Max F. Perutz Laboratories (MFPL) are a center established by the University of Vienna and the Medical University of Vienna to provide an environment for excellent, internationally recognized research and education in the field of Molecular Biology. Currently, the MFPL host around 60 independent research groups, involving around 500 people from over 40 nations.
Scientific contacts
Ass. Prof. Dr. Alipasha Vaziri
Research Institute of Molecular Pathology (IMP)
Max F. Perutz Laboratories (MFPL) and Research Platform Quantum Phenomena & Nanoscale Biological Systems (QuNaBioS), University of Vienna
T +43-1-79730-3540
alipasha.vaziri(at)univie.ac.at
Dr. Manuel Zimmer
Research Institute of Molecular Pathology (IMP)
T +43-1-79730-3430
manuel.zimmer(at)imp.ac.at
Press contact
Dr. Lilly Sommer
Max F. Perutz Laboratories
Communications
T +43-1-4277-240 14
lilly.sommer(at)univie.ac.a
Wissenschaftlicher Kontakt
Ass. Prof. Dr. Alipasha Vaziri
Max F. Perutz Laboratories (MFPL) and Research Platform Quantum Phenomena & Nanoscale Biological Systems (QuNaBioS)Universität Wien
+43-1-79730-35 40
+43-664-60277-542 52
alipasha.vaziri@univie.ac.at
Rückfragehinweis
Dr. Lilly Sommer
Max F. Perutz Laboratories, CommunicationsUniversität Wien
1030 - Wien, Dr.-Bohr-Gasse 9
+43-1-4277-240 14
lilly.sommer@univie.ac.at
Downloads:
Vaziri_NatureMethods_01.jpg
Dateigröße: 751,05 KB
