Unraveling truly one dimensional carbon solids
04. April 2016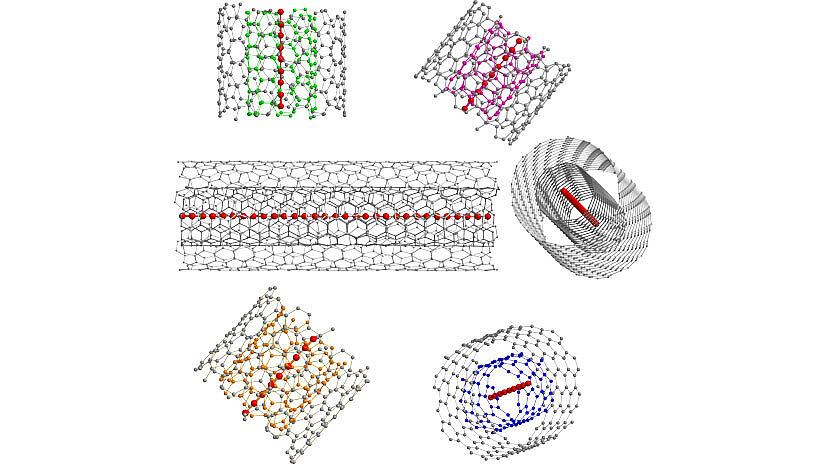
Schematic representation of confined ultra-long acetylenic linear carbon chains inside different double walled carbon nanotubes (Copyright: Lei Shi, Faculty of Physics, University of Vienna).
Direct proof of stable ultra-long 1D carbon chains as a route to carbyne
Elemental carbon appears in many different forms, including diamond and graphite. Their unique structural, electrical and optical properties have a broad range of potential applications in composite materials and nanoelectronics. Within the “carbon family”, only carbyne, the truly one-dimensional form of carbon, has not yet been synthesized; although studied for the last 50 years, its extreme instability in ambient conditions has rendered the final experimental proof of its existence elusive. In an international collaboration, researchers at the University of Vienna, led by Thomas Pichler, have succeeded in developing a novel route for the bulk production of carbon chains composed of more than 6,000 carbon atoms, using thin double-walled carbon nanotubes as protective hosts for the chains. These findings represent an elegant forerunner towards the final goal of carbyne’s bulk production and will be published in Nature Materials.
Even in its elemental form, the high bond versatility of carbon allows for many different well-known materials, including diamond and graphite. A single layer of graphite, termed graphene, can then be rolled or folded into carbon nanotubes or fullerenes, respectively. To date, Nobel prizes have been awarded for both graphene (2010) and fullerenes (1996). Although the existence of carbyne, an infinitely long carbon chain, was proposed in 1885 by Adolf von Baeyer (Nobel laureate for his overall contributions in organic chemistry, 1905), scientists have not yet been able to synthesize this material. Von Baeyer even suggested that carbyne would remain elusive as its high reactivity would always lead to its immediate destruction. Nevertheless, carbon chains of increasing length have been successfully synthesized over the last 50 years, with a record of around 100 carbon atoms (2003). This record has now been broken by more than one order of magnitude, with the demonstration of micrometer length-scale chains.
The new record
Researchers from the University of Vienna, led by Thomas Pichler, have presented a novel approach to grow and stabilize carbon chains with a record length of 6,000 carbon atoms, improving the previous record by more than one order of magnitude. They use the confined space inside a double-walled carbon nanotube as a nano-reactor to grow ultra-long carbon chains on a bulk scale. In collaboration with the groups of Kazu Suenaga at the AIST Tsukuba in Japan, Lukas Novotny at the ETH Zürich in Switzerland and Angel Rubio at the MPI Hamburg in Germany and UPV/EHU San Sebastian in Spain, the existence of the chains has been unambiguously confirmed by using a multitude of sophisticated, complementary methods. These are temperature dependent near- and far-field Raman spectroscopy with different lasers (for the investigation of electronic and vibrational properties), high resolution transmission electron spectroscopy (for the direct observation of carbyne inside the carbon nanotubes) and x-ray scattering (for the confirmation of bulk chain growth).
The researchers present their study in the latest edition of "Nature Materials". "The direct experimental proof of confined ultra-long linear carbon chains, which are more than an order of magnitude longer than the longest proven chains so far, can be seen as a promising step towards the final goal of unraveling the “holy grail” of carbon allotropes, carbyne”, explains the lead author, Lei Shi.
Application potential
Carbyne is very stable inside double-walled carbon nanotubes. This property is crucial for its eventual application in future materials and devices. According to theoretical models, carbyne’s mechanical properties exceed all known materials, outperforming both graphene and diamond. Carbyne’s electrical properties suggest novel nanoelectronic applications in quantum spin transport and magnetic semiconductors.
The work was supported by FWF and the EU.
Publication in "Nature Materials":
"Confined linear carbon chains as a route to bulk carbyne": Lei Shi, Philip Rohringer, Kazu Suenaga, Yoshiko Niimi,Jani Kotakoski, Jannik C. Meyer, Herwig Peterlik, Marius Wanko, Seymur Cahangirov, Angel Rubio, Zachary J. Lapin, Lukas Novotny, Paola Ayala, Thomas Pichler, Nature Materials, 2016
http://dx.doi.org/10.1038/NMAT4617
http://arxiv.org/1507.04896
Wissenschaftlicher Kontakt
Univ.-Prof. Mag. Dr. Thomas Pichler
Elektronische Materialeigenschaften Fakultät für PhysikUniversität Wien
1090 - Wien, Boltzmanngasse 5
+43-664-60277-514 66
thomas.pichler@univie.ac.at
Rückfragehinweis
Mag. Alexandra Frey
Media Relations ManagerUniversität Wien
1010 - Wien, Universitätsring 1
+43-1-4277-17533
+43-664-8175675
alexandra.frey@univie.ac.at
Downloads:
Carbin_01.png
Dateigröße: 9,3 MB
