A brilliant solution
16. Juni 2015Scientists from the University of Vienna improve light directed Chemistry
A team of scientists from the Department of Inorganic Chemistry, the Department of Nutritional and Physiological Chemistry of the University of Vienna, and the Christian Doppler Laboratory for Bioactive Aroma Compounds, led by Mark Somoza and Veronika Somoza, together with a colleague at Roche Diagnostics, have introduced a powerful new molecular tool for directing chemical reactions using light. The advance will be useful in fields ranging from optogenetics to drug discovery and development. The results appeared last week as a "Hot Paper" in the leading chemical journal "Angewandte Chemie".
Modern chemical synthesis is capable of producing extraordinarily complex molecules and arrangements of molecules. This capability is one of the foundations of almost any modern technological innovation, from the manufacture of consumer products to the synthesis of pharmaceuticals and other advanced substances and materials.
Chemists direct the progress of complex syntheses by promoting reactions at certain molecular positions while blocking reactions at others. Reactions are blocked by attaching chemical protecting groups that can later be removed to allow subsequent reactions to take place or to reveal the finished product. Chemical blocking groups are designed to be removed under specific and easily controllable conditions, such as basic or acidic conditions. Blocking groups that can be removed by exposure to light are much more versatile, allowing both very precise spatial and temporal control of reactions.
These photolabile groups are widely used in high technology applications, such as high-resolution 3D laser printing, photolithographic synthesis of DNA microarrays for genomics experiments, and photoactivatable bioactive molecules used to study living cells. Every-day industrial uses include inks, adhesives and coatings that can be hardened instantly by exposure to ultraviolet light. In recent years dentists have also relied on this technology, in the form of photopolymer composites, to replace the mercury alloy traditionally used in fillings.
Much research has been devoted over the years to develop new photolabile groups that can be removed cleanly, quickly, and with a minimum of light. However, progress has been slow because it is not currently possible to design, theoretically or computationally, better photolabile groups.
Next-Generation Photolabile Groups
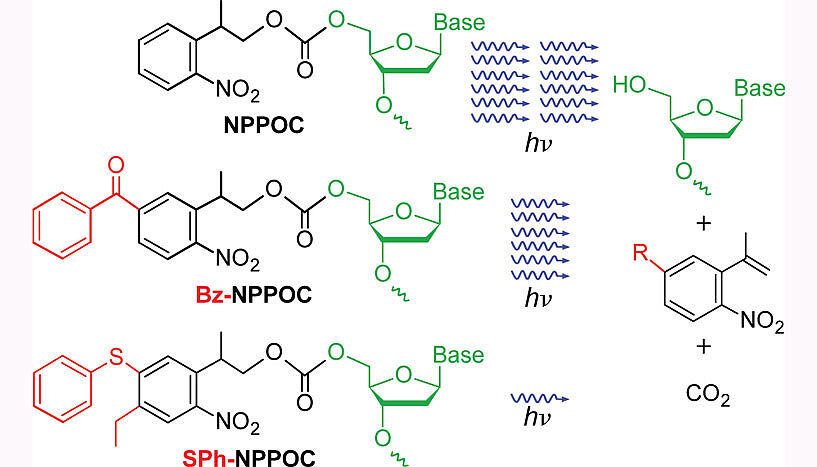
The most useful and commonly used photolabile groups are the nitrobenzyl type of molecule, but these need a lot of light in order to be activated, and therefore require long illumination times or very bright light sources. The new results published in Angewandte Chemie show that two nitrobenzyl-type molecules, Bz-NPPOC and SPh-NPPOC are much better. They need one-half, or one-twelfth, respectively, of the amount of light needed by the older generation of these molecules. SPh-NPPOC, in particular, is an almost perfect photolabile group because it is both very sensitive to light and is a good match to the commonly used light sources, mercury lamps, and the newest solid-state light sources, both UV-LEDs and diode lasers. Solid state sources have tremendous advantages in terms of flexibility and reliability, but even high-power UV-LEDs cannot match the intensity of mercury arc lamps; however the efficiency of SPh-NPPOC will allow the use of these new light sources in most applications.
The greatly improved photolabile groups should lead to their widespread use in both research and commercial applications.
Publication in "Angewandte Chemie International Edition":
Nicole Kretschy, Ann-Katrin Holik, Veronika Somoza, Klaus-Peter Stengele, and
Mark M. Somoza: Next-Generation Photolabile o-Nitrobenzyl Groups for Light-Directed
Chemistry and Microarray Synthesis. In: Angewandte Chemie International Edition (June 2015).
DOI: http://dx.doi.org/10.1002/anie.201502125
Wissenschaftlicher Kontakt
Assoz. Prof. Mark Somoza, Privatdoz. B.Sc. MSc PhD
Institut für Anorganische ChemieUniversität Wien
1090 - Wien, Währinger Straße 42
+43-1-4277-52643
mark.somoza@univie.ac.at
Rückfragehinweis
Stephan Brodicky
Pressebüro der Universität WienUniversität Wien
1010 - Wien, Universitätsring 1
+43-1-4277-175 41
+43-664-60277-175 41
stephan.brodicky@univie.ac.at
Downloads:
teamphoto_01.jpg
Dateigröße: 2,32 MB